Your What does reduction mean in chemistry images are ready. What does reduction mean in chemistry are a topic that is being searched for and liked by netizens now. You can Get the What does reduction mean in chemistry files here. Get all free photos.
If you’re looking for what does reduction mean in chemistry pictures information linked to the what does reduction mean in chemistry topic, you have pay a visit to the right blog. Our site frequently provides you with suggestions for seeing the highest quality video and picture content, please kindly hunt and find more informative video articles and images that match your interests.
What Does Reduction Mean In Chemistry. A type of chemical reaction in which oxidation and reduction occurs is called a redox reaction which stands for reduction-oxidation. Which of the following reactions does not involve oxidation-reduction. Your TV uses 120VAC and your dryer or electric stove uses 240vac. Harm reduction is an approach to treating those with alcohol and other substance-use problems that does not require patients to commit to complete abstinence before.
 Difference Between Oxidation And Reduction Redox Reaction Class 11 Electrochemistry 01 Youtube From youtube.com
Difference Between Oxidation And Reduction Redox Reaction Class 11 Electrochemistry 01 Youtube From youtube.com
An atom becomes an ion or a charged atom because of the gain or loss of electrons. The answer there requires diving into physics and chemistry. The reduced species receives electrons whereas the oxidised species loses them. Chemistry 2e is designed to meet the scope and sequence requirements of the two-semester general chemistry course. There is a lot of confusion about the Hueckel rule. A reduction-oxidation or redox reaction is a type of chemical reaction in which reduction and oxidation occur at the same time.
Throughout the chapters David.
Your TV uses 120VAC and your dryer or electric stove uses 240vac. Thanx for this post. Whether youre in high school or already in college the following guide will help you compose an excellent chemistry paper. Balancing oxidation-reduction reactions also known as redox reactions using the half-reaction method in acidic and basic solutions. Chemistry is Everywhere Introductory Chemistry is intended for a one-semester introductory or preparatory chemistry course. The book also includes a number of innovative features.
 Source: chemistry.stackexchange.com
Source: chemistry.stackexchange.com
A type of chemical reaction in which oxidation and reduction occurs is called a redox reaction which stands for reduction-oxidation. 120vac is the accepted standard of. The residence time of a fluid parcel is the total time that the parcel has spent inside a control volume eg. Reduction ALLOW from 0 to 1 in HI 0 in ALLOW Iodine oxidised from 0 to 1 AND 3 Ior 2 for iodine numbers around equation for oxidation states 1 for 1 AND 1 for 1 NOTE for iodineI 2 from 0 only needs to be seen once does not need to be stated twice 1 mark for 3 ox nos correct but no mention of words oxidationreduction. Whether youre in high school or already in college the following guide will help you compose an excellent chemistry paper.
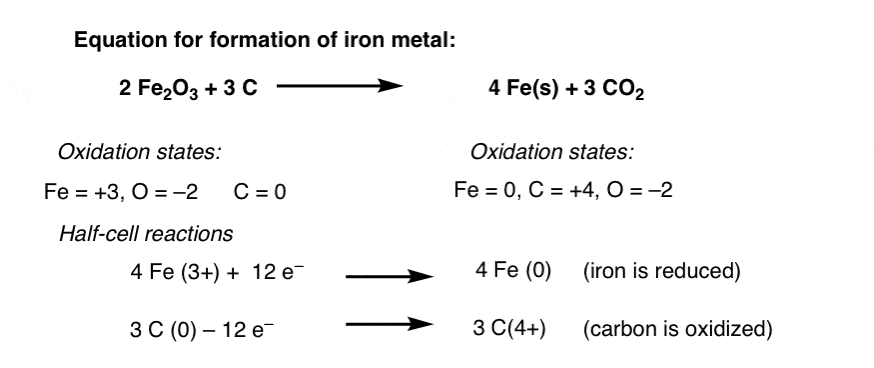 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
The book also includes a number of innovative features. It does not automatically mean an explosive one. When the Earth comes out of an ice age the warming is not initiated by CO2 but by changes in the Earths orbit. Tips on How to Write a Chemistry Paper. Negative ions are larger than their source atom because of the gain of an electron.
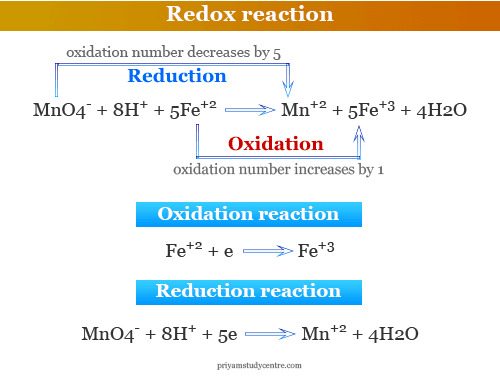 Source: priyamstudycentre.com
Source: priyamstudycentre.com
The thermal gradient in the atmosphere is a consequence of the gradual reduction in pressure as the height increases arising from the action of gravity. Throughout the chapters David. An atom becomes an ion or a charged atom because of the gain or loss of electrons. Negative ions are larger than their source atom because of the gain of an electron. But how exactly do certain molecules trap heat.
 Source: pinterest.com
Source: pinterest.com
Reduction ALLOW from 0 to 1 in HI 0 in ALLOW Iodine oxidised from 0 to 1 AND 3 Ior 2 for iodine numbers around equation for oxidation states 1 for 1 AND 1 for 1 NOTE for iodineI 2 from 0 only needs to be seen once does not need to be stated twice 1 mark for 3 ox nos correct but no mention of words oxidationreduction. Harm reduction is an approach to treating those with alcohol and other substance-use problems that does not require patients to commit to complete abstinence before. The second-highest level of detail is the structural formula which replaces those dots with lines. When the Earth comes out of an ice age the warming is not initiated by CO2 but by changes in the Earths orbit. Chemistry 2e is designed to meet the scope and sequence requirements of the two-semester general chemistry course.
 Source: chemistrylearner.com
Source: chemistrylearner.com
Reduction ALLOW from 0 to 1 in HI 0 in ALLOW Iodine oxidised from 0 to 1 AND 3 Ior 2 for iodine numbers around equation for oxidation states 1 for 1 AND 1 for 1 NOTE for iodineI 2 from 0 only needs to be seen once does not need to be stated twice 1 mark for 3 ox nos correct but no mention of words oxidationreduction. Tips on How to Write a Chemistry Paper. All are oxidation-reactions A 159-g sample of a metal chloride MCl2 is dissolved in. Each subject has its own rules when it comes to writing papers. A reduction-oxidation or redox reaction is a type of chemical reaction in which reduction and oxidation occur at the same time.

The book also includes a number of innovative features. The oxidation of a metal by oxygen gas could then be explained as the metal atom losing electrons to form the cation being oxidized with the oxygen molecule gaining electrons to form oxygen anions. Its a significant and harmful consequence of excess carbon dioxide in the atmosphere that we dont see or feel because its effects are happening underwater. A type of chemical reaction in which oxidation and reduction occurs is called a redox reaction which stands for reduction-oxidation. The anode where oxidation occurs and the cathode where reduction occurs note that Cathode does not mean and Anode does not mean - Volt meter.
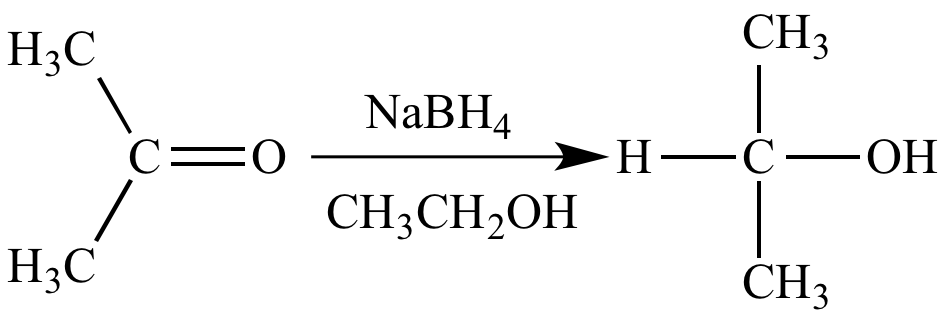 Source: chem.ucla.edu
Source: chem.ucla.edu
Balancing oxidation-reduction reactions also known as redox reactions using the half-reaction method in acidic and basic solutions. I personally like azulene more for the 10-electron example as each individual ring does not conform the Hueckel rule while your example still has to separate rings with each 6 π electrons. Electrochemistry and electrochemical cells. Chemistry is Everywhere Introductory Chemistry is intended for a one-semester introductory or preparatory chemistry course. The third level of detail is the line.
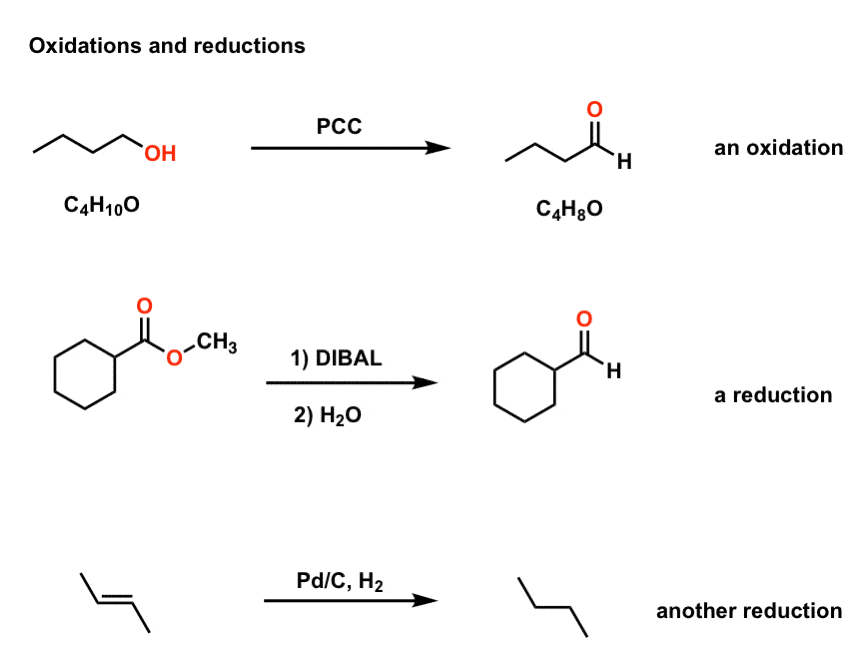 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
A lit desk lamp on a dark desk in a dark room. Chemical reaction a process in which one or more substances the reactants are converted to one or more different substances the productsSubstances are either chemical elements or compoundsA chemical reaction rearranges the constituent atoms of the reactants to create different substances as products. An atom becomes an ion or a charged atom because of the gain or loss of electrons. More down to earth let us take the example below a simple dark scene. The thermal gradient in the atmosphere is a consequence of the gradual reduction in pressure as the height increases arising from the action of gravity.
 Source: en.wikiversity.org
Source: en.wikiversity.org
The standard reduction potential for a substance indicates how readily that substance gains electrons relative to other substances at standard conditions. Throughout the chapters David. Thats why the first step is to have your lab notes in order. The standard reduction potential for a substance indicates how readily that substance gains electrons relative to other substances at standard conditions. It does not automatically mean an explosive one.
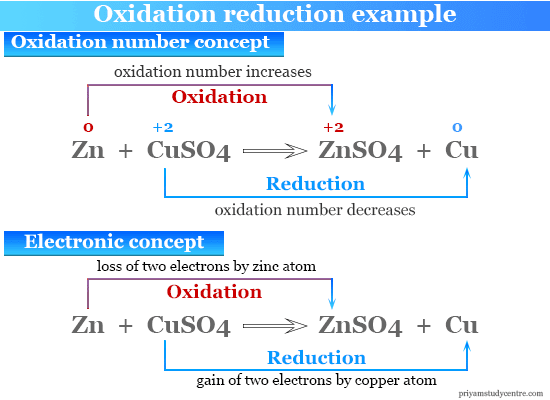 Source: priyamstudycentre.com
Source: priyamstudycentre.com
The developer acts more on the difference in the reduction speed of Silver contained in the exposed crystals versus Silver contained in the unexposed ones. Recall that there are at least 4 major ways of representing molecules that youre introduced to in the first week of ochem. Chemistry 2e is designed to meet the scope and sequence requirements of the two-semester general chemistry course. Each subject has its own rules when it comes to writing papers. Oxidizing agent and reducing agent.
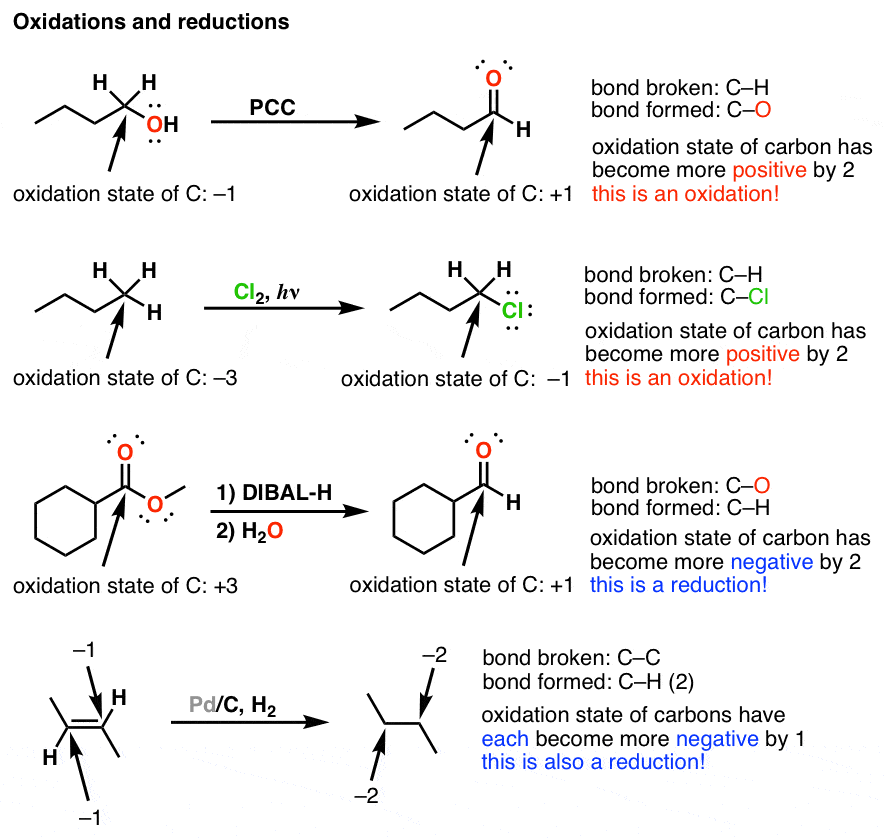 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
Oxidizing agent and reducing agent. In chemistry the organization is the key. 120vac is the accepted standard of. Recall that there are at least 4 major ways of representing molecules that youre introduced to in the first week of ochem. The developer acts more on the difference in the reduction speed of Silver contained in the exposed crystals versus Silver contained in the unexposed ones.
 Source: pinterest.com
Source: pinterest.com
Thats why the first step is to have your lab notes in order. It does not automatically mean an explosive one. The residence time of a fluid parcel is the total time that the parcel has spent inside a control volume eg. 120vac is the accepted standard of. Ionic size changes depending on the charge of the ion.
 Source: pinterest.com
Source: pinterest.com
The second-highest level of detail is the structural formula which replaces those dots with lines. I personally like azulene more for the 10-electron example as each individual ring does not conform the Hueckel rule while your example still has to separate rings with each 6 π electrons. The developer acts more on the difference in the reduction speed of Silver contained in the exposed crystals versus Silver contained in the unexposed ones. The warming causes the oceans to give up CO2. Chemical reaction a process in which one or more substances the reactants are converted to one or more different substances the productsSubstances are either chemical elements or compoundsA chemical reaction rearranges the constituent atoms of the reactants to create different substances as products.
 Source: youtube.com
Source: youtube.com
A type of chemical reaction in which oxidation and reduction occurs is called a redox reaction which stands for reduction-oxidation. Reduction-oxidation reactions are often called redox equations. An atom becomes an ion or a charged atom because of the gain or loss of electrons. A lit desk lamp on a dark desk in a dark room. More down to earth let us take the example below a simple dark scene.

All are oxidation-reactions A 159-g sample of a metal chloride MCl2 is dissolved in. Each subject has its own rules when it comes to writing papers. Negative ions are larger than their source atom because of the gain of an electron. When the Earth comes out of an ice age the warming is not initiated by CO2 but by changes in the Earths orbit. The second-highest level of detail is the structural formula which replaces those dots with lines.
 Source: youtube.com
Source: youtube.com
Positive ions are smaller than their source atom because of the loss of an electron which sometimes results in the loss of an ion ring. Reduction reactions always occur in conjunction with oxidation reactions in which a reactant loses one or more electrons. V volts a alternating c current vac is what the lights and appliances in your house use. Each subject has its own rules when it comes to writing papers. Reduction ALLOW from 0 to 1 in HI 0 in ALLOW Iodine oxidised from 0 to 1 AND 3 Ior 2 for iodine numbers around equation for oxidation states 1 for 1 AND 1 for 1 NOTE for iodineI 2 from 0 only needs to be seen once does not need to be stated twice 1 mark for 3 ox nos correct but no mention of words oxidationreduction.
 Source: masterorganicchemistry.com
Source: masterorganicchemistry.com
An oxidation process does not need the presence of oxygen despite its name. Does high insulin mean diabetes symptoms nhs. A type of chemical reaction in which oxidation and reduction occurs is called a redox reaction which stands for reduction-oxidation. The highest level of detail is the Lewis dot structure which shows where all the electrons are with dots. Negative ions are larger than their source atom because of the gain of an electron.
 Source: study.com
Source: study.com
By this mean now. Each subject has its own rules when it comes to writing papers. Niacin or nicotinic acid is a B vitamin that the body needs to help turn food into fuel. By this mean now. The oxidation of a metal by oxygen gas could then be explained as the metal atom losing electrons to form the cation being oxidized with the oxygen molecule gaining electrons to form oxygen anions.
This site is an open community for users to share their favorite wallpapers on the internet, all images or pictures in this website are for personal wallpaper use only, it is stricly prohibited to use this wallpaper for commercial purposes, if you are the author and find this image is shared without your permission, please kindly raise a DMCA report to Us.
If you find this site helpful, please support us by sharing this posts to your preference social media accounts like Facebook, Instagram and so on or you can also save this blog page with the title what does reduction mean in chemistry by using Ctrl + D for devices a laptop with a Windows operating system or Command + D for laptops with an Apple operating system. If you use a smartphone, you can also use the drawer menu of the browser you are using. Whether it’s a Windows, Mac, iOS or Android operating system, you will still be able to bookmark this website.






